Xenium stLearn CCI Gridding tutorial¶
This tutorial runs through stLearn CCI analysis on extremely large data, containing >166,000 single cells with gene expression measured in space.
To increase computation speed, we grid the cells and perform stLearn CCI analysis while taking into account the proportion of different cell types detected per grid.
Environment setup¶
[ ]:
import pandas as pd
import numpy as np
import stlearn as st
import scanpy as sc
import warnings
warnings.filterwarnings("ignore")
Loading the data¶
For this tutorial purpose, we don’t perform the clustering. Please run the clustering method with the clustering tutorial or a part of spatial trajectory inference tutorial.
[6]:
adata = sc.read_h5ad('xenium_louvain.h5ad')
[7]:
adata.X = adata.layers['raw_count'].copy()
adata.X
[7]:
array([[0., 3., 1., ..., 0., 6., 1.],
[0., 0., 0., ..., 0., 1., 0.],
[0., 0., 0., ..., 0., 0., 0.],
...,
[4., 1., 6., ..., 0., 0., 3.],
[1., 0., 0., ..., 0., 0., 0.],
[4., 1., 4., ..., 0., 0., 0.]], dtype=float32)
[8]:
st.pl.cluster_plot(adata, use_label="louvain", image_alpha=0, size=4, figsize=(10, 10))
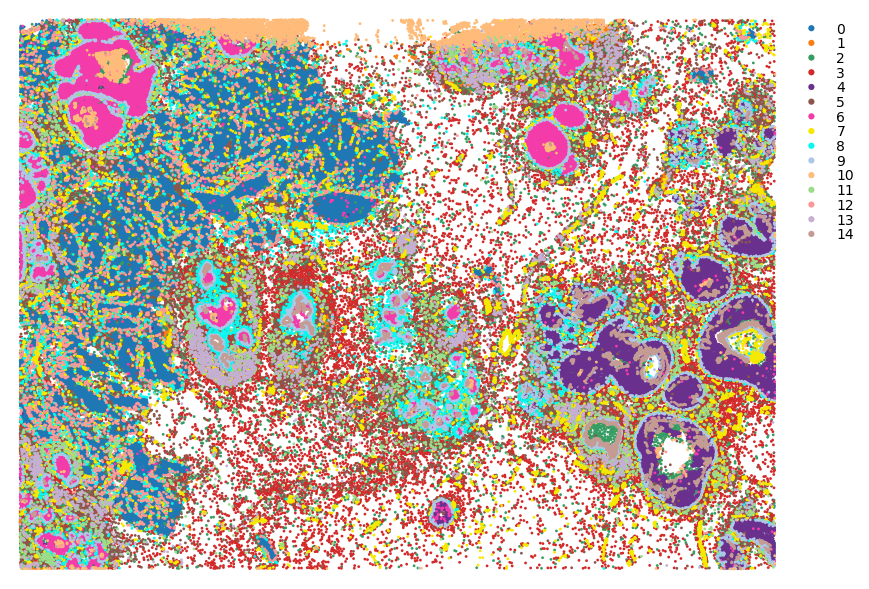
Note on normalisation¶
No log1p or shrinking to make genes of similar expression range. In our case, for calling hotspots, we want genes to be more separate, since we select background genes with similar expression levels to detect hotspots.
[9]:
#### Normalize total...
st.pp.normalize_total(adata)
adata.X
Normalization step is finished in adata.X
[9]:
array([[0. , 3.2922077 , 1.0974026 , ..., 0. , 6.5844154 ,
1.0974026 ],
[0. , 0. , 0. , ..., 0. , 2.640625 ,
0. ],
[0. , 0. , 0. , ..., 0. , 0. ,
0. ],
...,
[1.6650246 , 0.41625616, 2.497537 , ..., 0. , 0. ,
1.2487684 ],
[1.4083333 , 0. , 0. , ..., 0. , 0. ,
0. ],
[1.7201018 , 0.43002546, 1.7201018 , ..., 0. , 0. ,
0. ]], dtype=float32)
Gridding¶
Now performing the gridding. The resolution chosen here may effect the results. The higher resolution, the better this represents the single cell data but the longer the computation takes.
To summarise the gene expression across cells in a grid, we sum the library size normalised gene expression. Summing allows for representing the fact there are multiple cells in a given spot.
[10]:
### Calculating the number of grid spots we will generate
n_ = 125
print(f'{n_} by {n_} has this many spots:\n', n_*n_)
125 by 125 has this many spots:
15625
By providing ‘use_label’ to the function below, the cell type information is saved as deconvolution information per spot, and also the dominant cell annotation. That way we can perform stLearn CCI with the cell type information!
[11]:
### Gridding.
grid = st.tl.cci.grid(adata, n_row=n_, n_col=n_, use_label = 'louvain')
print( grid.shape ) # Slightly less than the above calculation, since we filter out spots with 0 cells.
Gridding...
(14521, 313)
Checking the gridding¶
Comparing the gridded data to the original, to make sure it makes sense.
It’s recommend to visualise the dominant cell types per spot, in order to gauge whether the tissue structure is adequately maintained after gridding (i.e. to make sure it is not too low resolution!).
[16]:
import matplotlib.pyplot as plt
fig, axes = plt.subplots(ncols=2, figsize=(20,8))
st.pl.cluster_plot(grid, use_label='louvain', size=10, ax=axes[0], show_plot=False)
st.pl.cluster_plot(adata, use_label='louvain', ax=axes[1], show_plot=False)
axes[0].set_title(f'Grid louvain dominant spots')
axes[1].set_title(f'Cell louvain labels')
plt.show()
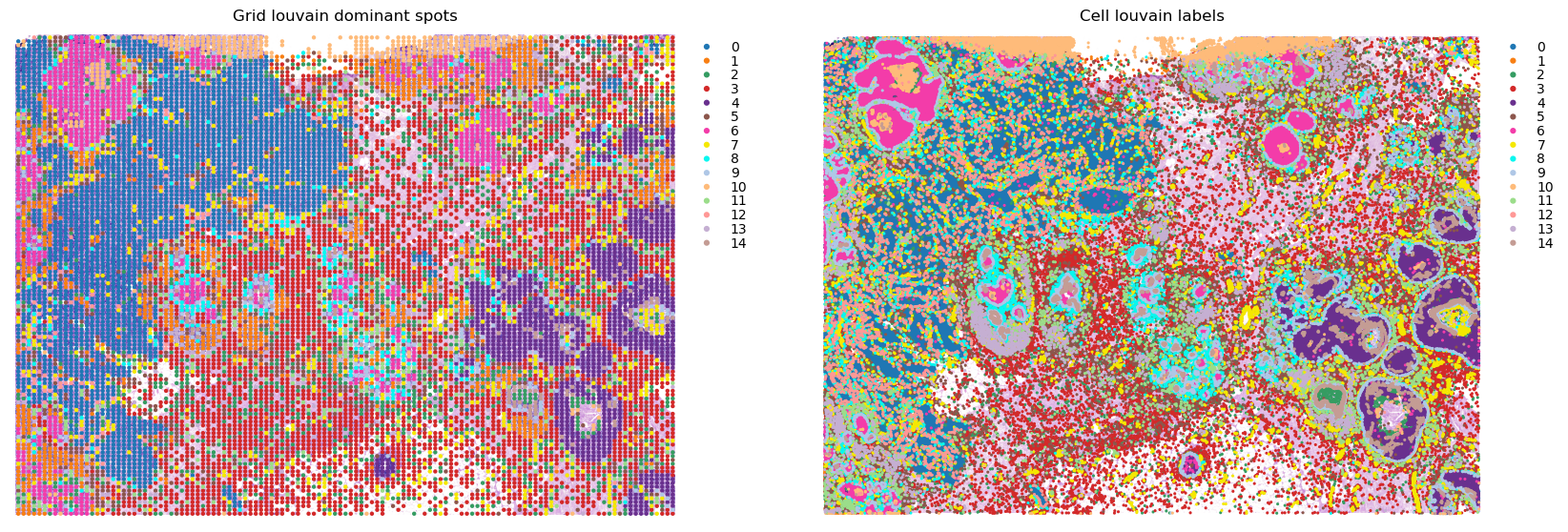
[24]:
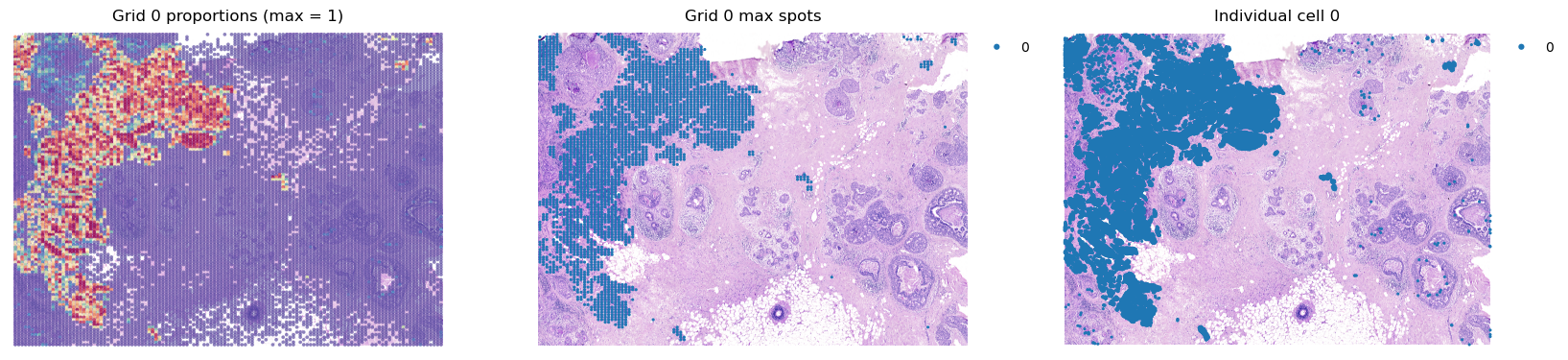
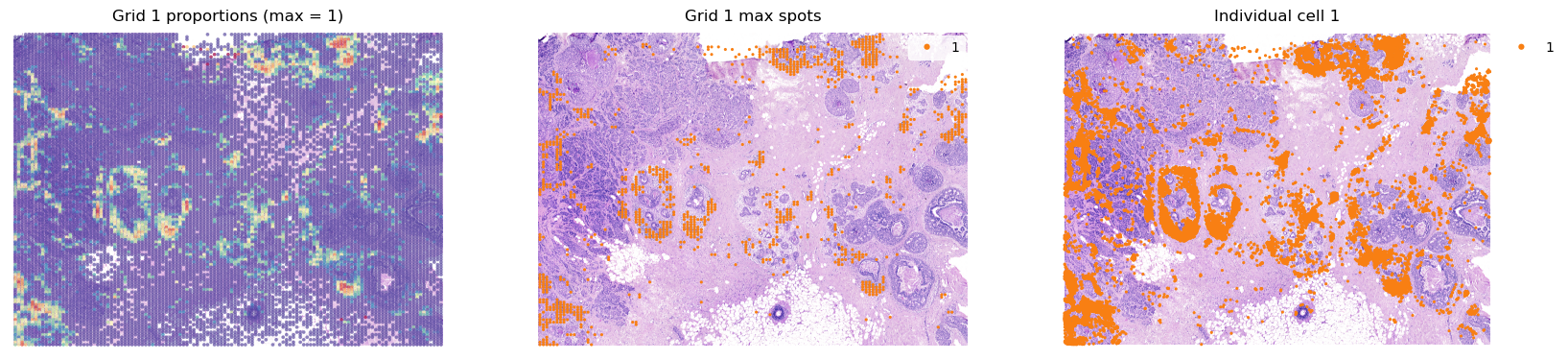
groups = list(grid.obs['louvain'].cat.categories)
for group in groups[0:2]:
fig, axes = plt.subplots(ncols=3, figsize=(20,8))
group_props = grid.uns['louvain'][group].values
grid.obs['group'] = group_props
st.pl.feat_plot(grid, feature='group', ax=axes[0], show_plot=False, vmax=1, show_color_bar=False)
st.pl.cluster_plot(grid, use_label='louvain', list_clusters=[group], ax=axes[1], show_plot=False)
st.pl.cluster_plot(adata, use_label='louvain', list_clusters=[group], ax=axes[2], show_plot=False)
axes[0].set_title(f'Grid {group} proportions (max = 1)')
axes[1].set_title(f'Grid {group} max spots')
axes[2].set_title(f'Individual cell {group}')
plt.show()
[28]:
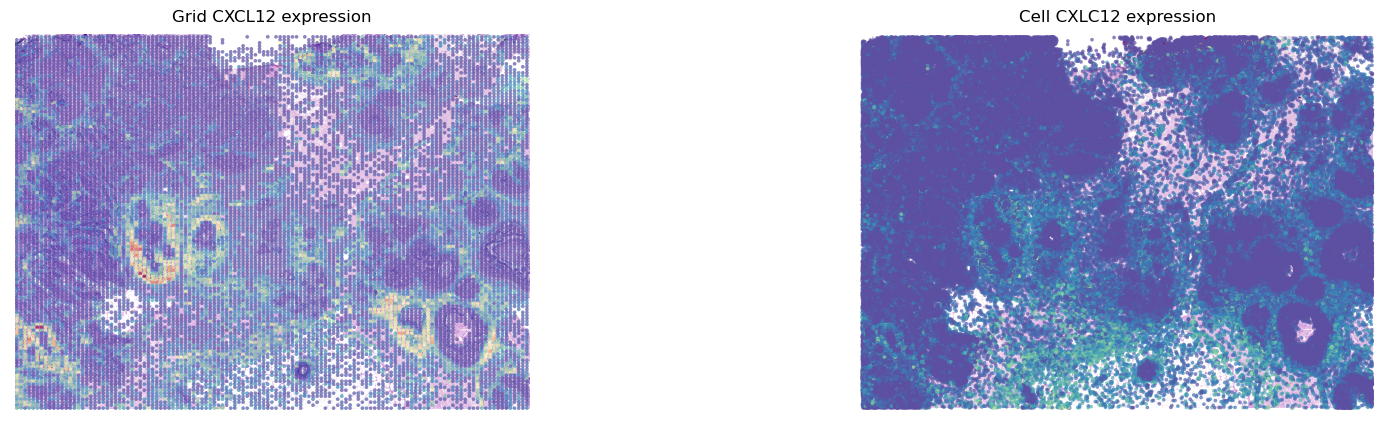
fig, axes = plt.subplots(ncols=2, figsize=(20,5))
st.pl.gene_plot(grid, gene_symbols='CXCL12', ax=axes[0],show_color_bar=False,show_plot=False)
st.pl.gene_plot(adata, gene_symbols='CXCL12', ax=axes[1], show_color_bar=False, show_plot=False,vmax=80)
axes[0].set_title(f'Grid CXCL12 expression')
axes[1].set_title(f'Cell CXLC12 expression')
plt.show()
LR Permutation Test¶
Running the LR permutation test, to determine regions of high LR co-expression.
[29]:
# Loading the LR databases available within stlearn (from NATMI)
lrs = st.tl.cci.load_lrs(['connectomeDB2020_lit'], species='human')
print(len(lrs))
2293
[31]:
# Running the analysis #
st.tl.cci.run(grid, lrs,
min_spots = 20, #Filter out any LR pairs with no scores for less than min_spots
distance=None, # None defaults to spot+immediate neighbours; distance=0 for within-spot mode
n_pairs=1000, # Number of random pairs to generate; low as example, recommend ~10,000
n_cpus=None, # Number of CPUs for parallel. If None, detects & use all available.
)
Calculating neighbours...
3 spots with no neighbours, 10 median spot neighbours.
Spot neighbour indices stored in adata.obsm['spot_neighbours'] & adata.obsm['spot_neigh_bcs'].
Altogether 20 valid L-R pairs
Generating backgrounds & testing each LR pair...: 100%|███████████████████████████████████████████ [ time left: 00:00 ]
Storing results:
lr_scores stored in adata.obsm['lr_scores'].
p_vals stored in adata.obsm['p_vals'].
p_adjs stored in adata.obsm['p_adjs'].
-log10(p_adjs) stored in adata.obsm['-log10(p_adjs)'].
lr_sig_scores stored in adata.obsm['lr_sig_scores'].
Per-spot results in adata.obsm have columns in same order as rows in adata.uns['lr_summary'].
Summary of LR results in adata.uns['lr_summary'].
[32]:
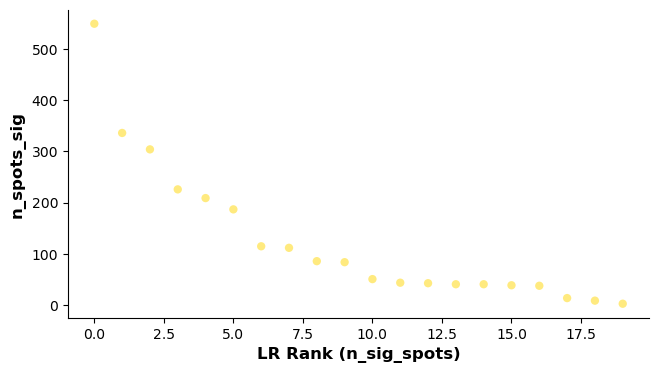
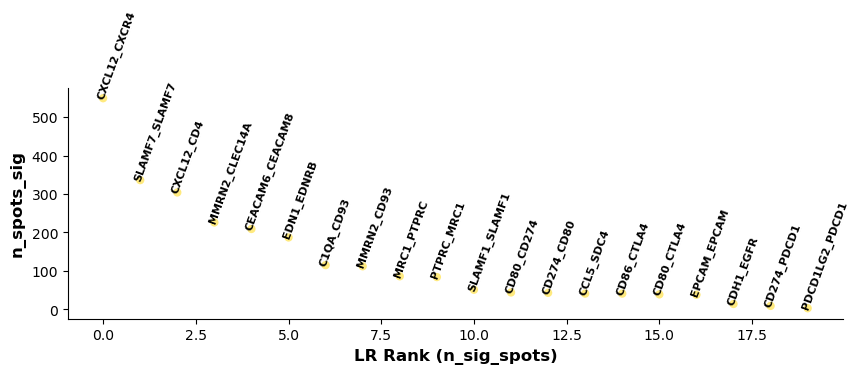
lr_info = grid.uns['lr_summary'] # A dataframe detailing the LR pairs ranked by number of significant spots.
print(lr_info.shape)
print(lr_info)
(20, 3)
n_spots n_spots_sig n_spots_sig_pval
CXCL12_CXCR4 14257 549 2548
SLAMF7_SLAMF7 8194 336 896
CXCL12_CD4 14094 304 3905
MMRN2_CLEC14A 7933 226 857
CEACAM6_CEACAM8 10689 209 1797
EDN1_EDNRB 8451 187 949
C1QA_CD93 12606 115 1042
MMRN2_CD93 10991 112 936
MRC1_PTPRC 13115 86 1157
PTPRC_MRC1 13115 84 1102
SLAMF1_SLAMF1 7591 51 394
CD80_CD274 4888 44 249
CD274_CD80 4888 43 254
CCL5_SDC4 12949 41 252
CD86_CTLA4 10209 41 279
CD80_CTLA4 6144 39 253
EPCAM_EPCAM 13371 38 2511
CDH1_EGFR 13718 14 385
CD274_PDCD1 1701 9 57
PDCD1LG2_PDCD1 1879 3 53
[33]:
# Showing the rankings of the LR from a global and local perspective.
# Ranking based on number of significant hotspots.
st.pl.lr_summary(grid, n_top=500)
st.pl.lr_summary(grid, n_top=50, figsize=(10,3))
[34]:
### Can adjust significance thresholds.
st.tl.cci.adj_pvals(grid, correct_axis='spot',
pval_adj_cutoff=0.05, adj_method='fdr_bh')
Updated adata.uns[lr_summary]
Updated adata.obsm[lr_scores]
Updated adata.obsm[lr_sig_scores]
Updated adata.obsm[p_vals]
Updated adata.obsm[p_adjs]
Updated adata.obsm[-log10(p_adjs)]
Downstream visualisations from LR analsyis¶
For more downstream visualisations, please see the stLearn CCI tutorial:
https://stlearn.readthedocs.io/en/latest/tutorials/stLearn-CCI.html
[35]:
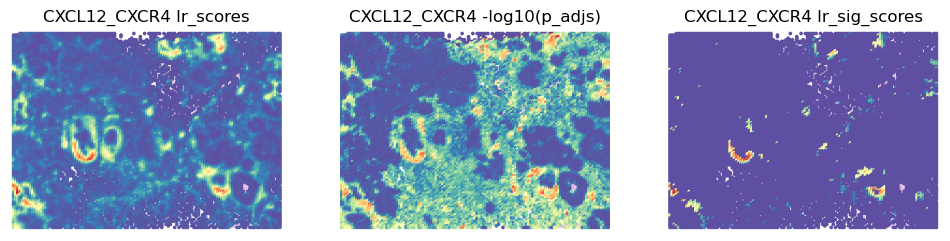
best_lr = grid.uns['lr_summary'].index.values[0] # Just choosing one of the top from lr_summary
[36]:
stats = ['lr_scores', '-log10(p_adjs)', 'lr_sig_scores']
fig, axes = plt.subplots(ncols=len(stats), figsize=(12,6))
for i, stat in enumerate(stats):
st.pl.lr_result_plot(grid, use_result=stat, use_lr=best_lr, show_color_bar=False, ax=axes[i])
axes[i].set_title(f'{best_lr} {stat}')
Predicting Significant Cell-Cell Interactions¶
For this analysis, we are using within-spot mode, with the deconvolution information which is stored in grid.uns, as automatically determined by st.tl.cci.grid. The permutation is performed to permute the cell proportions associated with each spot, to determine spots located in LR hotspots that have certain cell types over-represented.
[41]:
grid
[41]:
AnnData object with n_obs × n_vars = 14521 × 313
obs: 'imagecol', 'imagerow', 'n_cells', 'louvain', 'group'
uns: 'spatial', 'louvain', 'louvain_colors', 'grid_counts', 'grid_xedges', 'grid_yedges', 'lrfeatures', 'lr_summary'
obsm: 'spatial', 'spot_neighbours', 'spot_neigh_bcs', 'lr_scores', 'p_vals', 'p_adjs', '-log10(p_adjs)', 'lr_sig_scores'
[42]:
st.tl.cci.run_cci(grid, 'louvain', # Spot cell information either in data.obs or data.uns
min_spots=2, # Minimum number of spots for LR to be tested.
spot_mixtures=True, # If True will use the deconvolution data,
# so spots can have multiple cell types if score>cell_prop_cutoff
cell_prop_cutoff=0.1, # Spot considered to have cell type if score>0.1
sig_spots=True, # Only consider neighbourhoods of spots which had significant LR scores.
n_perms=100, # Permutations of cell information to get background, recommend ~1000
n_cpus=None,
)
Getting cached neighbourhood information...
Getting information for CCI counting...
Counting celltype-celltype interactions per LR and permutating 100 times.: 100%|██████████████████ [ time left: 00:00 ]
Significant counts of cci_rank interactions for all LR pairs in data.uns[lr_cci_louvain]
Significant counts of cci_rank interactions for each LR pair stored in dictionary data.uns[per_lr_cci_louvain]
[49]:
st.tl.cci.run_cci(grid, 'louvain', # Spot cell information either in data.obs or data.uns
min_spots=2, # Minimum number of spots for LR to be tested.
spot_mixtures=True, # If True will use the deconvolution data,
# so spots can have multiple cell types if score>cell_prop_cutoff
cell_prop_cutoff=0.1, # Spot considered to have cell type if score>0.1
sig_spots=True, # Only consider neighbourhoods of spots which had significant LR scores.
n_perms=100, # Permutations of cell information to get background, recommend ~1000
n_cpus=10,
)
Getting cached neighbourhood information...
Getting information for CCI counting...
Counting celltype-celltype interactions per LR and permutating 100 times.: 100%|███████████████████████████████████████████████████████████████████████████████████████████████████████ [ time left: 00:00 ]
Significant counts of cci_rank interactions for all LR pairs in data.uns[lr_cci_louvain]
Significant counts of cci_rank interactions for each LR pair stored in dictionary data.uns[per_lr_cci_louvain]
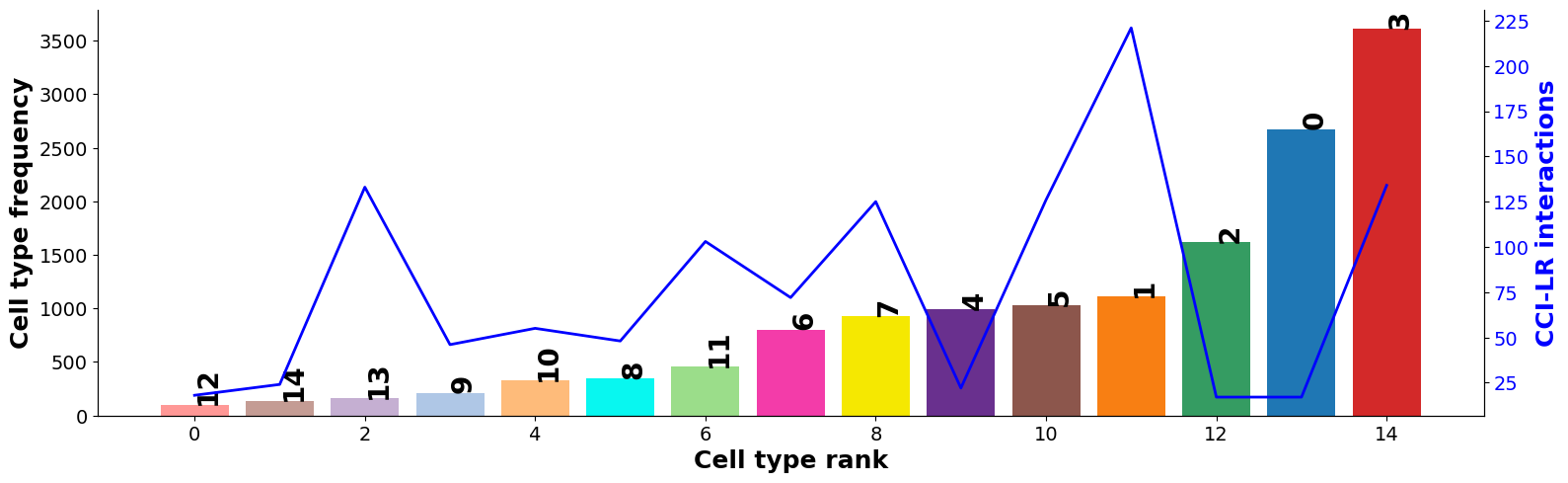
Diagnostic: checking for interaction and cell type frequency correlation¶
Should be little to no correlation; indicating the permutation has adequately controlled for cell type frequency.
[43]:
st.pl.cci_check(grid, 'louvain', figsize=(16,5))
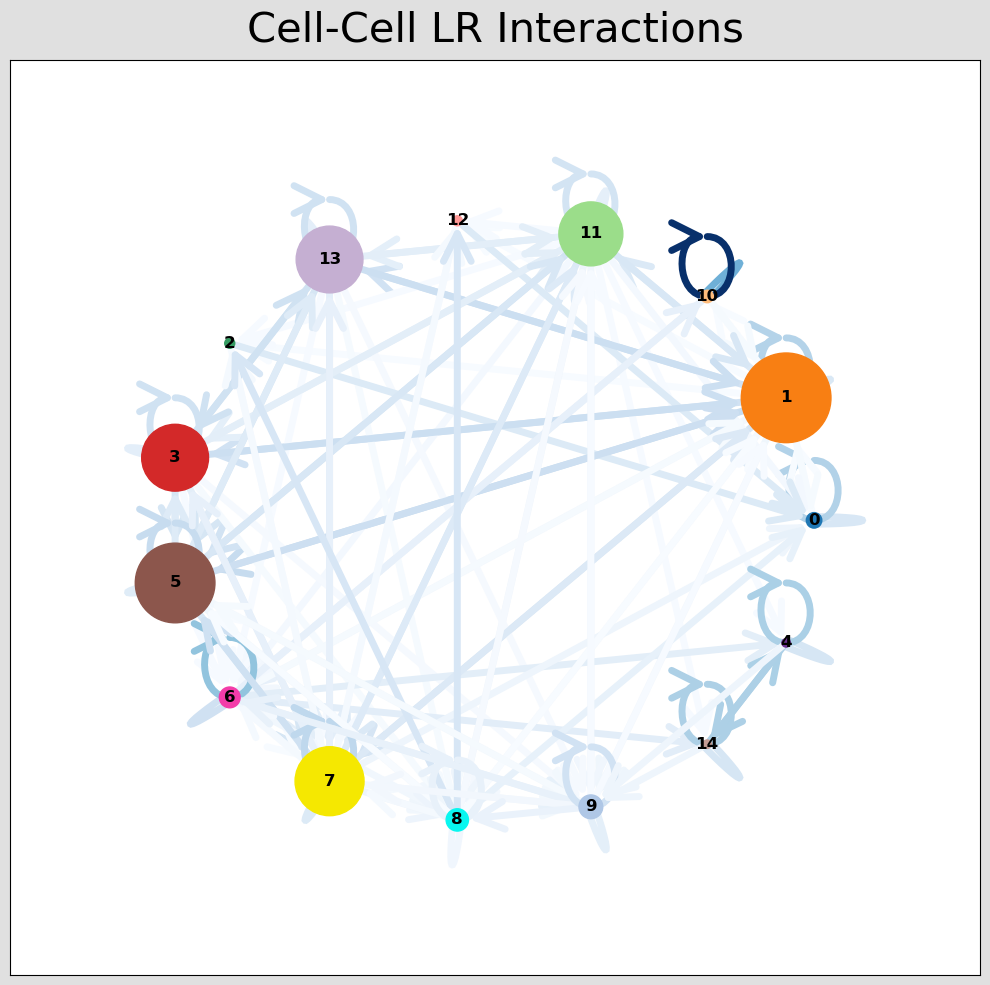
CCI Visualisations¶
CCI Network Plot¶
[44]:
# Visualising the no. of interactions between cell types across all LR pairs #
pos_1 = st.pl.ccinet_plot(grid, 'louvain', return_pos=True, min_counts=30)
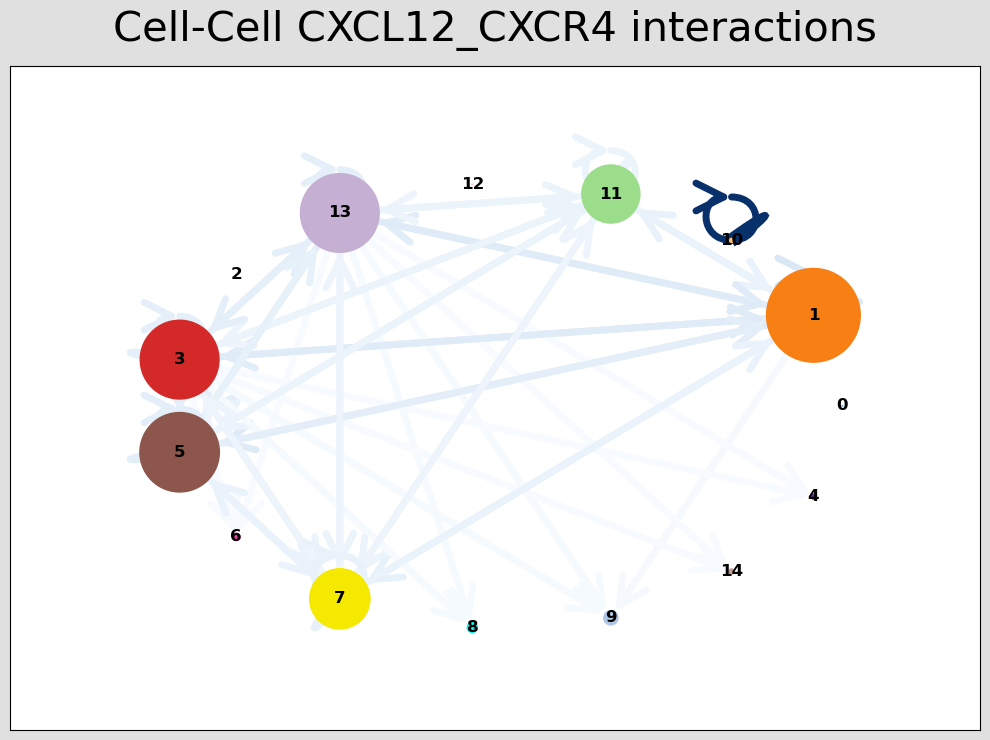
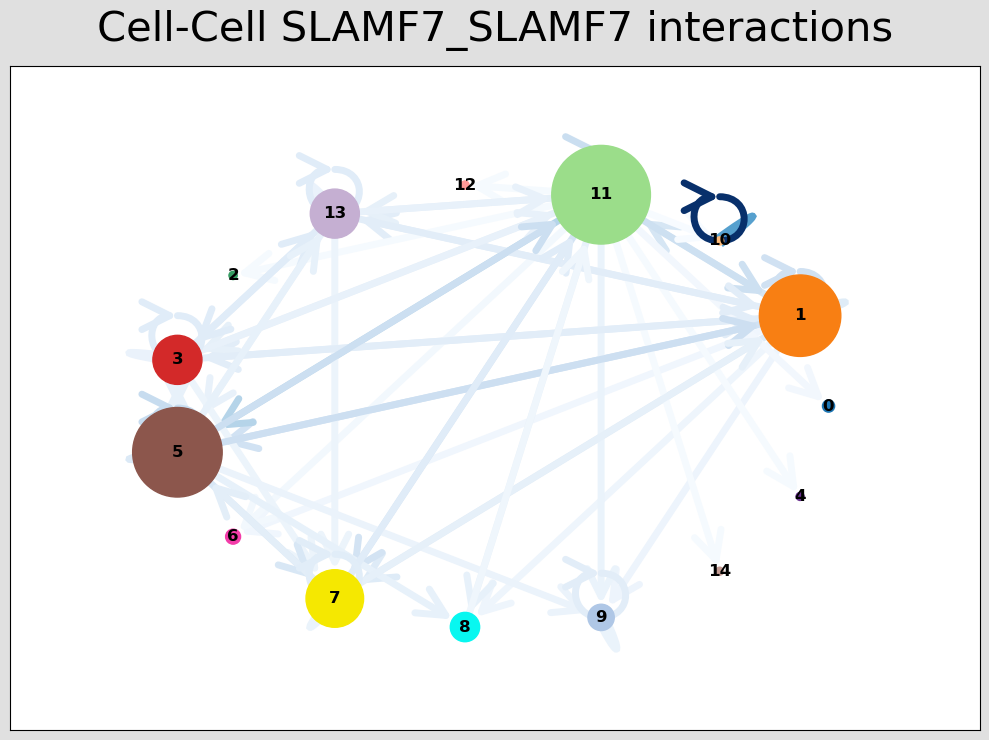
# Just examining the cell type interactions between selected pairs #
lrs = grid.uns['lr_summary'].index.values[0:3]
for best_lr in lrs[0:2]:
st.pl.ccinet_plot(grid, 'louvain', best_lr, min_counts=2,
figsize=(10,7.5), pos=pos_1,
)
CCI Chord Plot¶
[45]:
st.pl.lr_chord_plot(grid, 'louvain')
for lr in lrs[0:2]:
st.pl.lr_chord_plot(grid, 'louvain', lr)
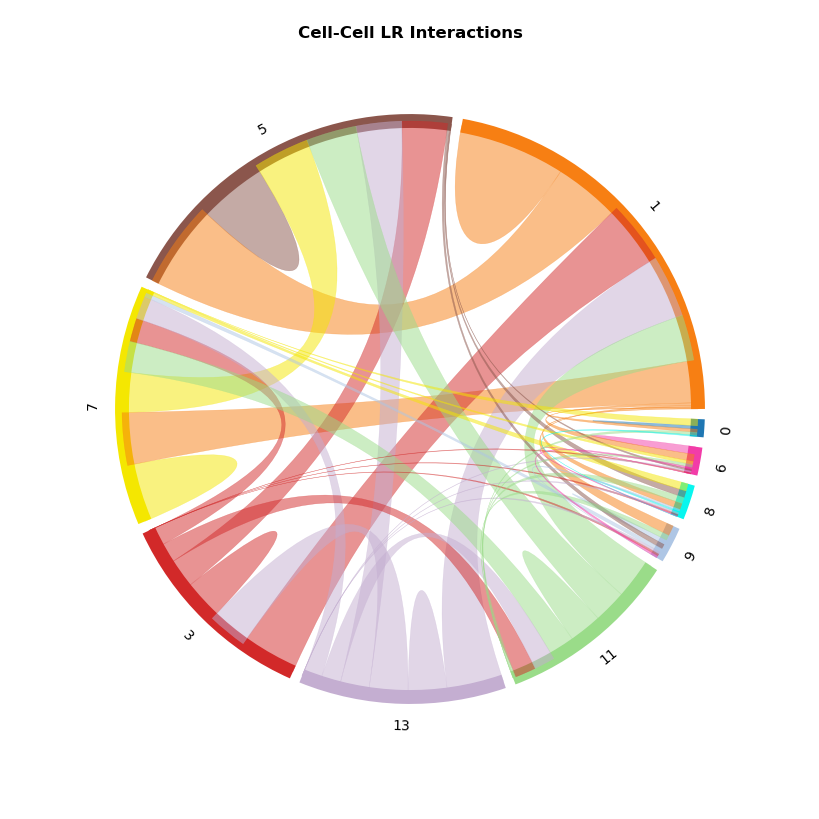
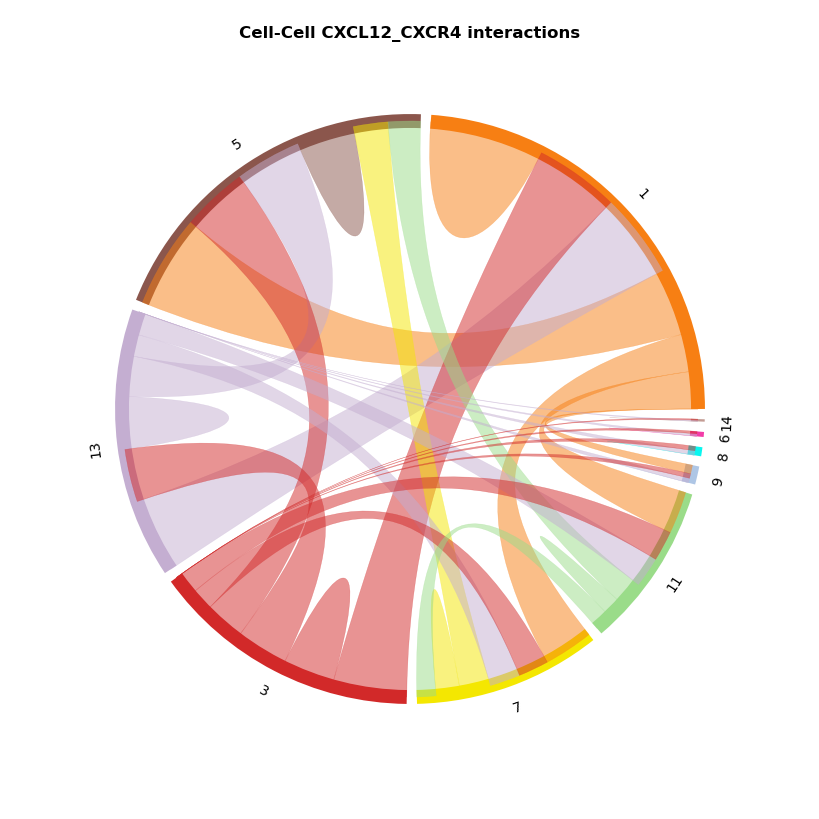
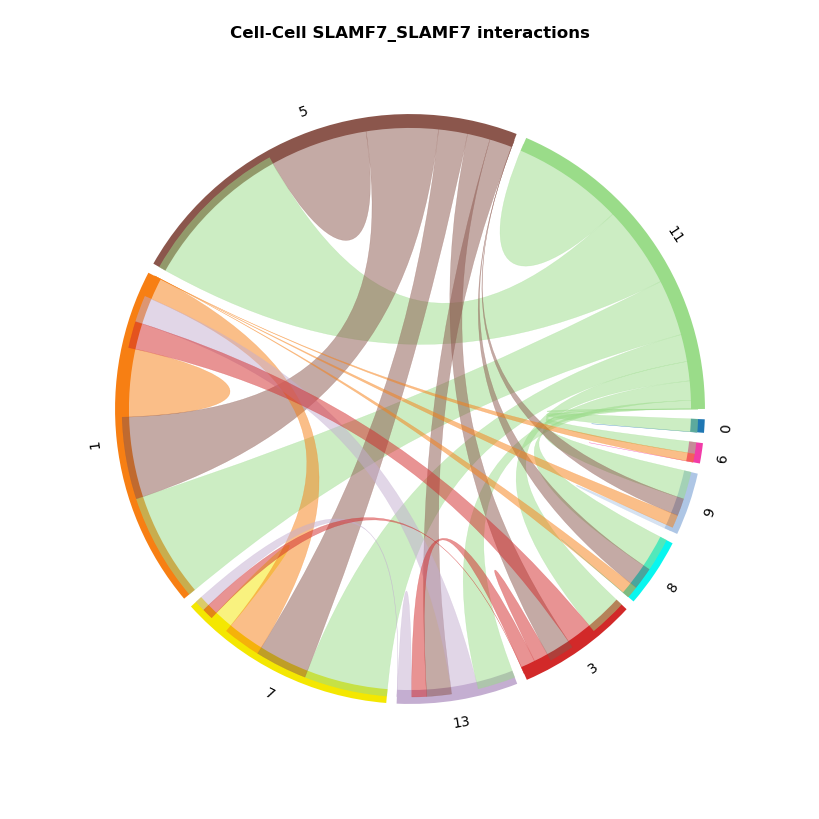
For additional visualisations and visualisation tips, please see:¶
https://stlearn.readthedocs.io/en/latest/tutorials/stLearn-CCI.html
Acknowledgement¶
We would like to thank Soo Hee Lee and 10X Genomics team for their able support, contribution and feedback to this tutorial